Poster Presentation First Malaria World Congress 2018
Relationship between relapse and weight-adjusted dose of primaquine in Korean vivax malaria (#487)
Aims: For radical cure of Korean vivax malaria, primaquine 15 mg base daily for 14 days is the standard regimen. However, this fixed dose of primaquine regimen might not meet the World Health Organization (WHO) guidance (0.25mg per kg per day) if the patients’ body weight exceed 60kg. The aims of this study were to 1) assess the relapse rate under the current standard primaquine regimen, and 2) reveal the risk factor for relapse in Korean vivax malaria.
Methods: We retrospectively reviewed the medical records of 1448 Korean vivax malaria patients from five general hospitals located in malaria endemic area between January 2000 and December 2016. Patients younger than 18 years old were excluded. Treatment consisted of hydroxychloroquine (2000 mg base administered over 3 days) plus primaquine (15 mg base/daily for 14 days). Patients were divided into two groups: relapse group and non-relapse group. We compared and statistically analyzed those two groups to reveal the risk factor for relapse. Furthermore, 24 health individuls 12 people with BMI less than 25 vs 12 people with BMI above 25) were recruited to analyze difference in primaquine pharmacokinetics.
Results: Among total 1448 cases, there were 41 relapsed cases and 1407 none-relapse. Overall, relapse rate of was 2.83%. There were significant differences between relapse vs. non-relapse groups in mean total administered dose of primaquine (0.21.±0.05 mg/kg vs. 0.25±0.09 mg/kg, p<0.001). Multivariate analysis identified older age (1.02(1.0-10.5), p=0.048), body weight exceeding 60kg (5.81(1.76-19.2), p=0.004), and primaquine dose under 0.25mg/kg/day (4.19(1.61-10.9), p=0.003) as risk factors. Pharmacokinetic analysis of primaquine in normal (BMI£ 25) vs obese (BMI>25) showed about 38% lower concentration in obese individuals.
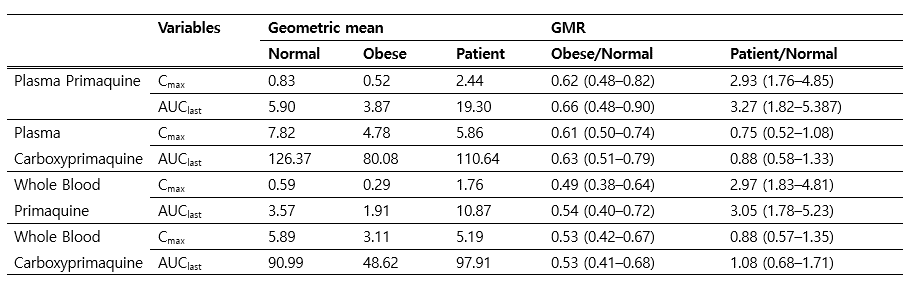
Conclusions: For radical cure of Korean vivax malaria, administration of 15mg base of primaquine regardless of body weight may pose higher risk of relapse.